Electrochemical “gap capacitance” published in J. Phys. Chem. Lett.
R. Sundararaman, M. C. Figueiredo, M. T. M. Koper and K. A. Schwarz, “Electrochemical Capacitance of CO-terminated Pt(111) is Dominated by CO-Solvent Gap”, J. Phys. Chem. Lett. 8, 5344 (2017)
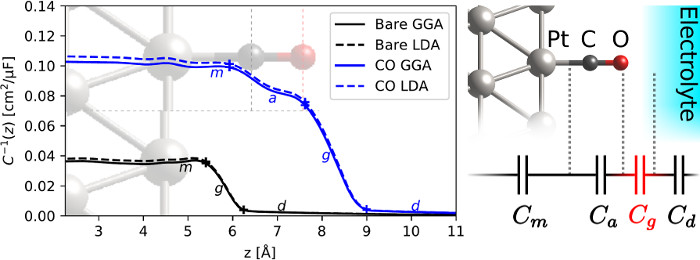
The electrochemical capacitance of metal surfaces with adsorbates such as carbon monoxide (CO) is substantially smaller than bare metal surfaces, and this was conventionally believed to be because these adsorbates act as an insulating spacer separating the metal from the electrolyte. In collaboration with Katie Schwarz at NIST and the Koper group at Leiden, we combined first-principles calculations using solvation models in JDFTx with experimental electrochemical capacitance measurements to show that, in fact, CO on platinum behaves like an extension of the metal! Instead, the insulation occurs after the CO adsorbate i.e. between the adsorbate and the electrolyte. Hydrophobic CO has a larger gap to the electrolyte compared to bare metal, and it is this gap capacitance that causes the observed low total capacitance in such systems. This understanding of the spatial distribution of electric fields and capacitance in electrochemical systems down to the molecular scale is critical for predicting and optimizing electrochemical reactions for energy conversion and storage.
