New solvation models for electrochemistry published in J. Chem. Phys.
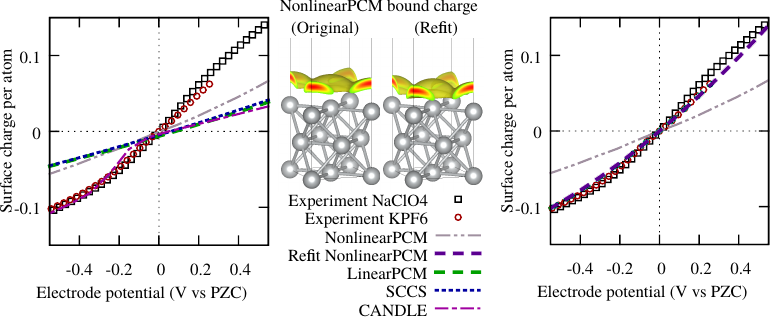
R. Sundararaman and K. Schwarz, “Evaluating continuum solvation models for the electrode-electrolyte interface: Challenges and strategies for improvement”, J. Chem. Phys. 146, 084111 (2017) (Preprint: arXiv:1612.00931)
Our team at NIST and RPI identified that continuum solvation models calibrated to experimental data for solvation energies of molecules do not necessarily extrapolate well to electrochemical interfaces. In particular, these models dramatically underestimate the capacitance of the electrochemical interface by almost a factor of two. We revise two popular solvation models to fix their capacitance predictions, without substantially altering their accuracy for molecular solvation energies.
